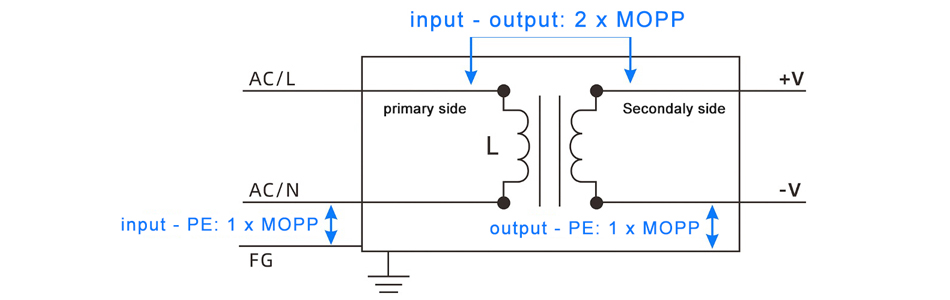
Featured posts





Latest posts

MEAN WELL continua a innovare nel settore dell’illuminazione intelligente con il lancio della serie XLC-25/40/60KN,...

Lifud presenta la serie CASAMBI ACB-B, una soluzione avanzata per il controllo wireless dell’illuminazione, perfetta...

Nel mondo dell’alimentazione industriale, l’ottimizzazione dello spazio e l’affidabilità dei dispositivi sono...

Siamo lieti di annunciare l'arrivo del nuovo modello Lifud LF-GBD150-6250-24, il driver LED di ultima generazione...

Nel mondo dell'illuminazione professionale, Lifud continua a distinguersi per l'innovazione e l'affidabilità. I nuovi...
Popular posts

![How to choose the DC AC Inverter [GUIDA 2023] How to choose the DC AC Inverter [GUIDA 2023]](https://www.alimentatorishop.com/img/ybc_blog/post/thumb/MINIATURA-NEWS.jpg)